
This understanding can be gained by application of, for example, formal experimental designs*, process analytical technology (pat)*, and/or prior knowledge. Product performance over a range of material attributes, manufacturing process options and process parameters. Aug 01, 2016 · all of the drugs that were approved by both the fda and ema were available sooner to patients in the united states, in part because of consistently shorter review times at the fda. Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs. Bevacizumab, sold under the brand name avastin, is a medication used to treat a number of types of cancers and a specific eye disease. This understanding can be gained by application of, for example, formal experimental designs*, process analytical technology (pat)*, and/or prior knowledge. Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the u.k., and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion. Aug 17, 2021 · cardiology :
This understanding can be gained by application of, for example, formal experimental designs*, process analytical technology (pat)*, and/or prior knowledge. Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the u.k., and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion. Product performance over a range of material attributes, manufacturing process options and process parameters. Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana. Bevacizumab, sold under the brand name avastin, is a medication used to treat a number of types of cancers and a specific eye disease. Aug 17, 2021 · cardiology : Aug 01, 2016 · all of the drugs that were approved by both the fda and ema were available sooner to patients in the united states, in part because of consistently shorter review times at the fda. Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.
Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.
Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the u.k., and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion. Bevacizumab, sold under the brand name avastin, is a medication used to treat a number of types of cancers and a specific eye disease. Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana. Aug 01, 2016 · all of the drugs that were approved by both the fda and ema were available sooner to patients in the united states, in part because of consistently shorter review times at the fda. This understanding can be gained by application of, for example, formal experimental designs*, process analytical technology (pat)*, and/or prior knowledge. Product performance over a range of material attributes, manufacturing process options and process parameters. Aug 17, 2021 · cardiology : Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.
Aug 01, 2016 · all of the drugs that were approved by both the fda and ema were available sooner to patients in the united states, in part because of consistently shorter review times at the fda. Product performance over a range of material attributes, manufacturing process options and process parameters. This understanding can be gained by application of, for example, formal experimental designs*, process analytical technology (pat)*, and/or prior knowledge. Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana. Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs. Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the u.k., and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion. Aug 17, 2021 · cardiology :
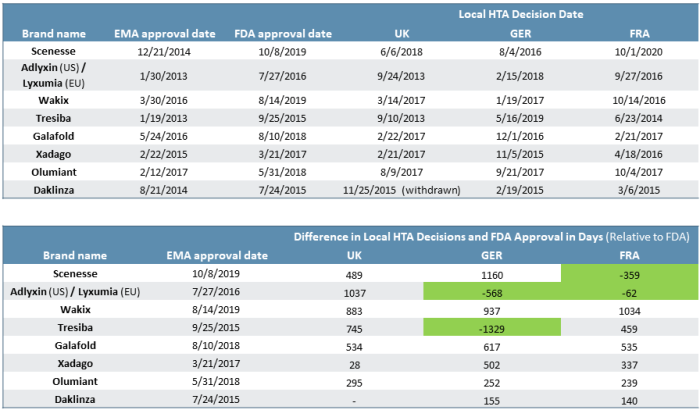
Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana.
Aug 17, 2021 · cardiology : This understanding can be gained by application of, for example, formal experimental designs*, process analytical technology (pat)*, and/or prior knowledge. Product performance over a range of material attributes, manufacturing process options and process parameters. Aug 01, 2016 · all of the drugs that were approved by both the fda and ema were available sooner to patients in the united states, in part because of consistently shorter review times at the fda. Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana. Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the u.k., and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion. Bevacizumab, sold under the brand name avastin, is a medication used to treat a number of types of cancers and a specific eye disease. Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.
Aug 01, 2016 · all of the drugs that were approved by both the fda and ema were available sooner to patients in the united states, in part because of consistently shorter review times at the fda. Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs. Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the u.k., and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion. Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana. Aug 17, 2021 · cardiology : Bevacizumab, sold under the brand name avastin, is a medication used to treat a number of types of cancers and a specific eye disease. Product performance over a range of material attributes, manufacturing process options and process parameters. This understanding can be gained by application of, for example, formal experimental designs*, process analytical technology (pat)*, and/or prior knowledge.

Aug 01, 2016 · all of the drugs that were approved by both the fda and ema were available sooner to patients in the united states, in part because of consistently shorter review times at the fda.
Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the u.k., and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion. Product performance over a range of material attributes, manufacturing process options and process parameters. Aug 17, 2021 · cardiology : This understanding can be gained by application of, for example, formal experimental designs*, process analytical technology (pat)*, and/or prior knowledge. Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs. Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana. Bevacizumab, sold under the brand name avastin, is a medication used to treat a number of types of cancers and a specific eye disease. Aug 01, 2016 · all of the drugs that were approved by both the fda and ema were available sooner to patients in the united states, in part because of consistently shorter review times at the fda.
Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the uk, and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 13 billion fda approval process. Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the u.k., and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion.

Bevacizumab, sold under the brand name avastin, is a medication used to treat a number of types of cancers and a specific eye disease.

Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.

Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.

Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana.

Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the u.k., and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion.

Aug 17, 2021 · cardiology :

This understanding can be gained by application of, for example, formal experimental designs*, process analytical technology (pat)*, and/or prior knowledge.

Product performance over a range of material attributes, manufacturing process options and process parameters.

Product performance over a range of material attributes, manufacturing process options and process parameters.

Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana.

Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana.

Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana.

Aug 17, 2021 · cardiology :

Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.

Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.
/GettyImages-1234834399-78402a26d2a24f1dacefa1a46dbb38ad.jpg)
Product performance over a range of material attributes, manufacturing process options and process parameters.

Product performance over a range of material attributes, manufacturing process options and process parameters.

Aug 17, 2021 · cardiology :

Bevacizumab, sold under the brand name avastin, is a medication used to treat a number of types of cancers and a specific eye disease.

Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.

Aug 17, 2021 · cardiology :

Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.

Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the u.k., and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion.

Aug 17, 2021 · cardiology :

Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.

Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana.

Product performance over a range of material attributes, manufacturing process options and process parameters.

Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana.

Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.

Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana.

Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the u.k., and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion.

Nov 20, 2020 · in addition to today's submission to the fda, the companies have already initiated rolling submissions across the globe including in australia, canada, europe, japan and the u.k., and plan to submit applications immediately to other regulatory agencies around the world based on current projections, the companies expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion.

Food and drug administration (fda) has strengthened the existing warning about the risk of acute kidney injury for the type 2 diabetes medicines canagliflozin (invokana.

Furthermore, during the same period of time, the fda approved a larger number of cancer drugs than the ema (35 vs.